This is an experimental blogpost.
It is not required reading -- feel free to skip it. I have chosen to write about xenon isotopes because it's an interesting challenge for a technical writer. Actually, it's two challenges. The first is to understand the bloody subject, and the second is to write about the bloody subject with enough clarity and wit to make other people understand it. Those of you who decide to read on will be rewarded with speculation about nuclear warfare on Mars. Thrills! So here we go...
An isotopic topic
The 118 elements in the periodic table are uniquely defined by their
atomic numbers, and this invariantly refers to the number of protons in the nucleus of an atom of that element. Hydrogen 1, Helium 2, Lithium 3...bla bla... Platinum 78, Gold 79, Mercury 80 ... bla bla .... Ununoctium 118, and there may well be a 119th and 120th coming along shortly, although only the first 94 elements are ever found naturally. The high-faluting ones are synthesized at vast expense and typically last for milliseconds. Only three atoms of Ununoctium have ever been synthesized. If you ask "Why would they do that?" their answer would be "
Because now we know." By the way, Ununoctium officially became an element only four months ago. How time doesn't fly.
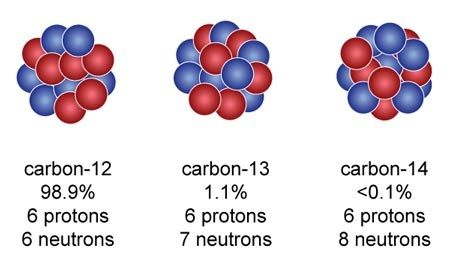
Counting protons does not tell us all about the nuclear structure of an element, because in almost every case the nucleus is made of neutrons as well as protons. Take carbon (please...) Its atomic number is 6, so its nucleus always has 6 protons by definition. But 98.9% of the carbon on Earth also has 6 neutrons in its nucleus. 6 + 6 = 12, so 12 is what is now called the
mass number of normal, bog standard carbon. Chemists know it by its nickname,
12C
6. But about 1% of carbon atoms you might find lying around have one extra neutron, and a very small proportion have two extra neutrons. They are, of course,
13C
6 and
14C
6 respectively, and the three different carbons are the
isotopes of carbon.
credit: emaze
Now we get to the radioactive part. Put a teaspoonful of carbon-12 or carbon-13 on your kitchen table, and they'll stay right there for ever. They are
stable isotopes. Even if you walk away and come back in a million years, there will still be exactly the same number of atoms on the table (unless you've had a fall of soot.) Carbon-14, however, is not like that. It is unstable, and spontaneously decays. Radioactive decay takes several forms but in this case each decay event causes a neutron to turn into a proton. The mass number thus remains 14, but the atomic number goes up one, and therefore that atom is no longer carbon. It becomes the next element in the table, which happens to be nitrogen. This decay process has a half-life of 5,730 years, so after that length of time half your carbon-14 has turned into nitrogen-14 and literally vanished into thin air. Nitrogen itself has one other stable isotope, nitrogen-15, plus a raft of unstable ones. Some of these decay back to carbon, some go on up to the next element, which happens to be oxygen.
So there's yer basic tute on what isotopes are and what mischief they get up to. Enter xenon.
Noble xenon
Helium, neon, argon, krypton, xenon, and radon are the six so-called "noble" gases, although -- isn't this interesting? -- the brand new element Ununoctium may well turn out to be an ultra-heavy noble gas. The big feature of the nobles is that they don't form bonds with other elements, unlike carbon which will make molecules with anything that asks, the slut. So there's no such thing as argon hydroxide or xenon hydrochloride, for example. Xenon has the distinction of having more stable isotopes than any other element except tin. Here's a reference to 13 xenon isotopes, eight of which are stable. "NA" means "Natural Abundance."
====================================
124Xe - NA 0.095%, decays to
124Te, half-life 5 x 10
16 y (essentially stable)
125Xe - NA zero, decays to
125I, half-life 16.9h
126Xe - NA 0.089%, stable
127Xe - NA zero, decays to
127I, half-life 36.345d
128Xe - NA 1.91%, stable
129Xe - NA 26.4%, stable. Produced by beta decay of
129I, half-life 16 million y
130Xe - NA 4.07%, stable
131Xe - NA 21.2%, stable. Fission product of Thorium,
Uranium, and Plutonium. Can be a decay product of
131I or
131Cs
132Xe - NA 26.9%, stable.
The most common natural isotope. Can be a decay product of
132I or
132Ba
133Xe - NA zero, decays to
133Cs, half-life 5.25d
134Xe - NA 10.4%, decays to
134Ba, half-life 1.1 x 10
16 y (essentially stable). Fission product of Thorium, Uranium, and Plutonium
135Xe - NA zero, decays to
135Cs, half-life 9.14h. Fission product of Thorium, Uranium, and Plutonium
136Xe - NA 8.86%, decays to
136Ba, half-life 2.2 x 10
21 y (essentially stable). Fission product of Thorium, Uranium, and Plutonium
====================================
The four isotopes that are fission products can be expected to be unusually plentiful in the aftermath of a nuclear fission weapon detonation, and indeed these isotopes have been used to detect weapon tests. Xenon-129 is not a fission product in the conventional sense, but it is generated by a special nuclear reaction called
fast fission. Fast fission is used in advanced reactor designs and in some fusion weapons (H-bombs, to put it crudely.)
Interplanetary warfare?
As shown in the above reference, in planet Earth's atmosphere, xenon 129 is only slightly less abundant than the dominant isotope 132 -- 26.4% cf. 26.9%. Thanks to some
brilliant space science, we now know a good deal about the atmosphere of planet Mars, and in that atmosphere 129 is more than 2.5 times as abundant as 132 (although both, of course, are far less abundant in absolute terms than the terrestrials.) This has led a maverick physicist called John Brandenburg to speculate that at least one fast fission event -- such as the detonation of a thermo-nuclear weapon -- has occurred at some time in Mars' history. Brandenburg has attracted quite a bit of attention by publishing this conjecture -- he's become a favorite on the pseudoscience circuit that includes
late-night radio chat shows, internet radio, and TV productions with a very lenient view of what constitutes a historical fact (Yes,
Ancient Aliens,
I'm talking about you.)
Figure from John Brandenburg's 2015 LPSC poster. NOTE that these data are relative, not absolute
So is Brandenburg right? Was Mars nearly obliterated by nuclear warfare? I hesitate to go up against a well-qualified physicist but my answer is probably not.
Very probably not. The thing is, you don't need such a radical hypothesis as fast fission to explain the 129/132 isotope ratio on Mars. Look at that reference table again. Xenon-129 is remarkable among the isotopes in that it can be, and is, created by decay of Iodine-129 with a half life of 16 million years. Is Iodine-129 itself a fission product? No it is not. It is, however, a solid, not a gas, at normal temperatures (boiling point 184.3 °C.) So if, let's say, Mars' primordial atmosphere was almost entirely lost due to some catastrophe early in its history, iodine would not be affected and it would not be strange that xenon-129 would be preferentially replenished.
Well, that's exactly what Marsologists say happened, some time within the first 100 million years after the planet was formed. The planet has been around for some 4.5 billion years, so there has been plenty of time for iodine-129 to quietly turn itself into xenon-129, even given that 16m year half-life. Iodine-132 also decays to xenon-132, but that isotope is extremely rare and the decay half-life is measured in hours, so it's irrelevant.
Wrap-up
NASA gets way too much scorn these days from people who are too ignorant to know better than to believe the chief NASA-haters, Richard Hoagland and Mike Bara. The agency is accused of fudging and masking important data, and avoiding key questions about extraterrestrial life. The truth, from what I know and have observed, is that NASA might delay release of some planetary data in order to give priority to the science teams to peer-review and publish them, but there is absolutely no concerted policy to hide evidence of living things or dodgy guesswork like nuclear explosions on Mars.
There is too much scorn, and not nearly enough gratitude for the brilliance of the JPL designers whose experiments make it possible to have these arguments.
With grateful thanks to my excellent research assistant, Google Search.
Update 8 May 2016
Element 118 has now been provisionally named
Oganesson (Og), honoring Yuri Oganessian who led the team that synthesised element-117.
=====================================
References:
Evidence for large, anomalous, nuclear explosions on Mars in the past. --Brandenburg, J.E. 46th Lunar and Planetary Science Conference (2105) poster session
Rational wikipedia article on Dr John Brandenburg
Was Mars murdered? Podcast shownotes by Dr Stuart Robbins,
Wikipedia article on xenon and its isotopes
Wikipedia article on Martian atmosphere
Fission product tables for typical heavy isotopes. IAEA document
USGS Isotope resource - Xenon